Epizyme Announces the U.S. Food and Drug Administration Lifts Partial Clinical Hold on Tazemetostat Clinical Program | Business Wire

EZH2 inhibitor tazemetostat in patients with relapsed or refractory, BAP1-inactivated malignant pleural mesothelioma: a multicentre, open-label, phase 2 study - The Lancet Oncology

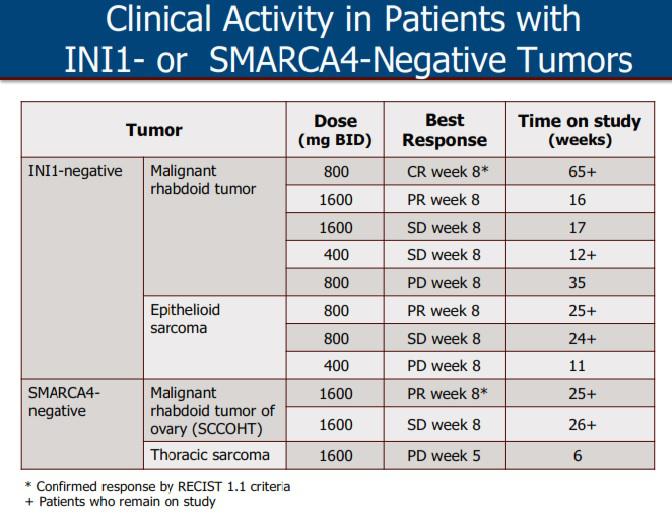
Epizyme Announces U.S. FDA Accelerated Approval of TAZVERIK™ (tazemetostat) for the Treatment of Patients with Epithelioid Sarcoma | Business Wire

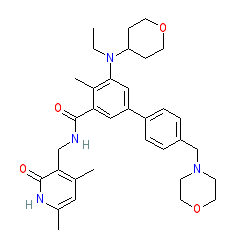
Targeting Enhancer of Zeste Homolog 2 for the Treatment of Hematological Malignancies and Solid Tumors: Candidate Structure–Activity Relationships Insights and Evolution Prospects | Journal of Medicinal Chemistry
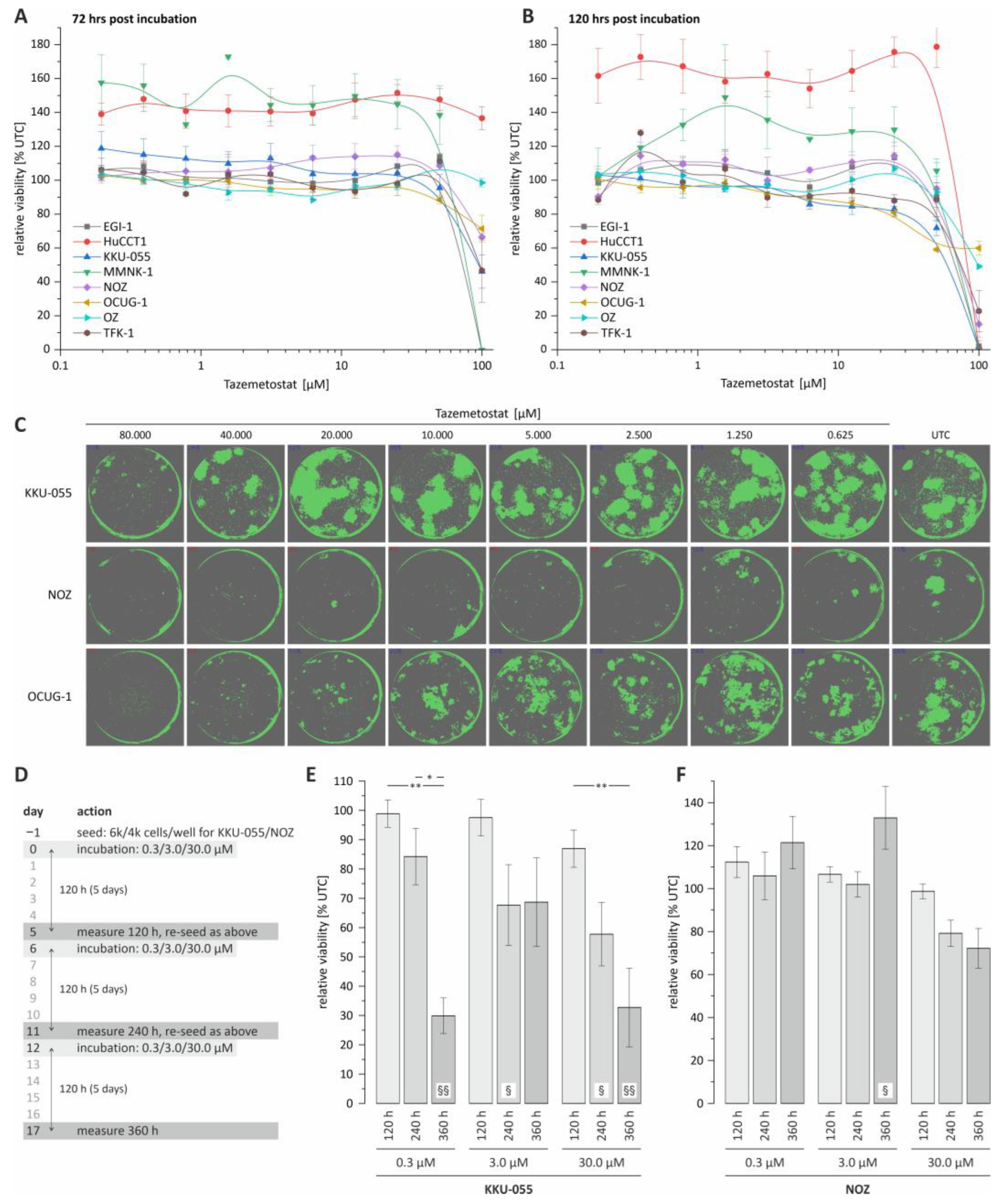
Cancers | Free Full-Text | Evaluation of Tazemetostat as a Therapeutically Relevant Substance in Biliary Tract Cancer

Tazemetostat Is Associated with Lower Risk for Safety Outcomes Versus the PI3-Kinases Idelalisib, Duvelisib and Copanlisib, in Patients with Relapsed/Refractory Follicular Lymphoma Who Have Received at Least 2 Prior Systemic Treatments: A

Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial - The Lancet Oncology

Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial - The Lancet Oncology

The EZH2 inhibitor tazemetostat upregulates the expression of CCL17/TARC in B‐cell lymphoma and enhances T‐cell recruitment - Yuan - 2021 - Cancer Science - Wiley Online Library

Testing the Addition of Tazemetostat to the Immunotherapy Drug, Pembrolizumab (MK-3475), in Advanced Urothelial Carcinoma

OncLive.com on Twitter: "Watch our upcoming live broadcast reviewing a new indication, clinical trial data, and dosing for TAZVERIK TM (tazemetostat): https://t.co/YKnSN20tj9 https://t.co/ZfU6XQc4Gd" / Twitter
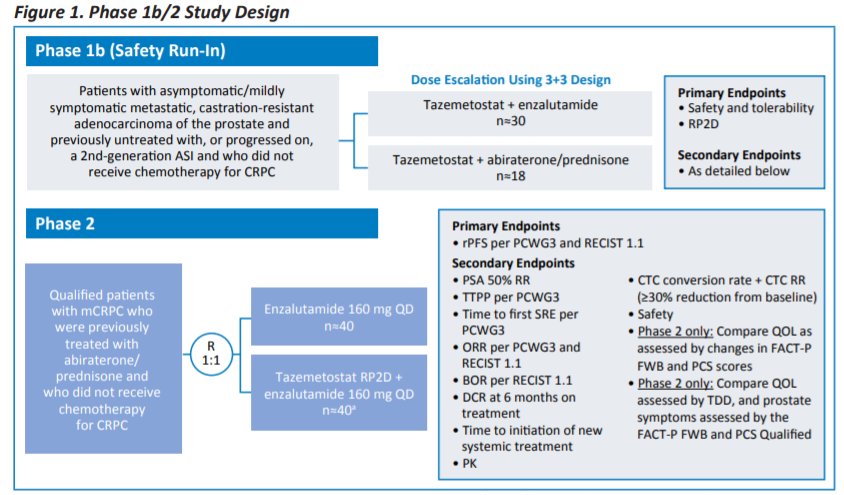
ESMO 2021: Safety of Tazemetostat in Combination With Abiraterone/Prednisone or Enzalutamide in Patients With Metastatic Castration-Resistant Prostate Cancer

Discovery of a Novel Covalent EZH2 Inhibitor Based on Tazemetostat Scaffold for the Treatment of Ovarian Cancer | Journal of Medicinal Chemistry
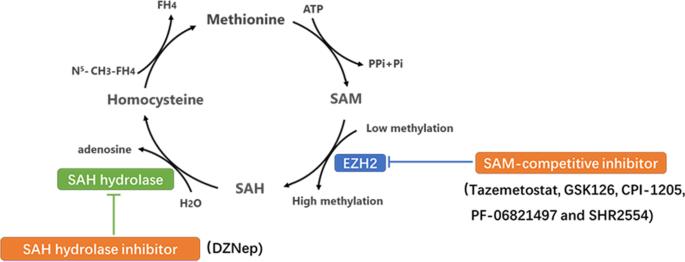
Finding an easy way to harmonize: a review of advances in clinical research and combination strategies of EZH2 inhibitors | Clinical Epigenetics | Full Text

Cancers | Free Full-Text | Evaluation of Tazemetostat as a Therapeutically Relevant Substance in Biliary Tract Cancer