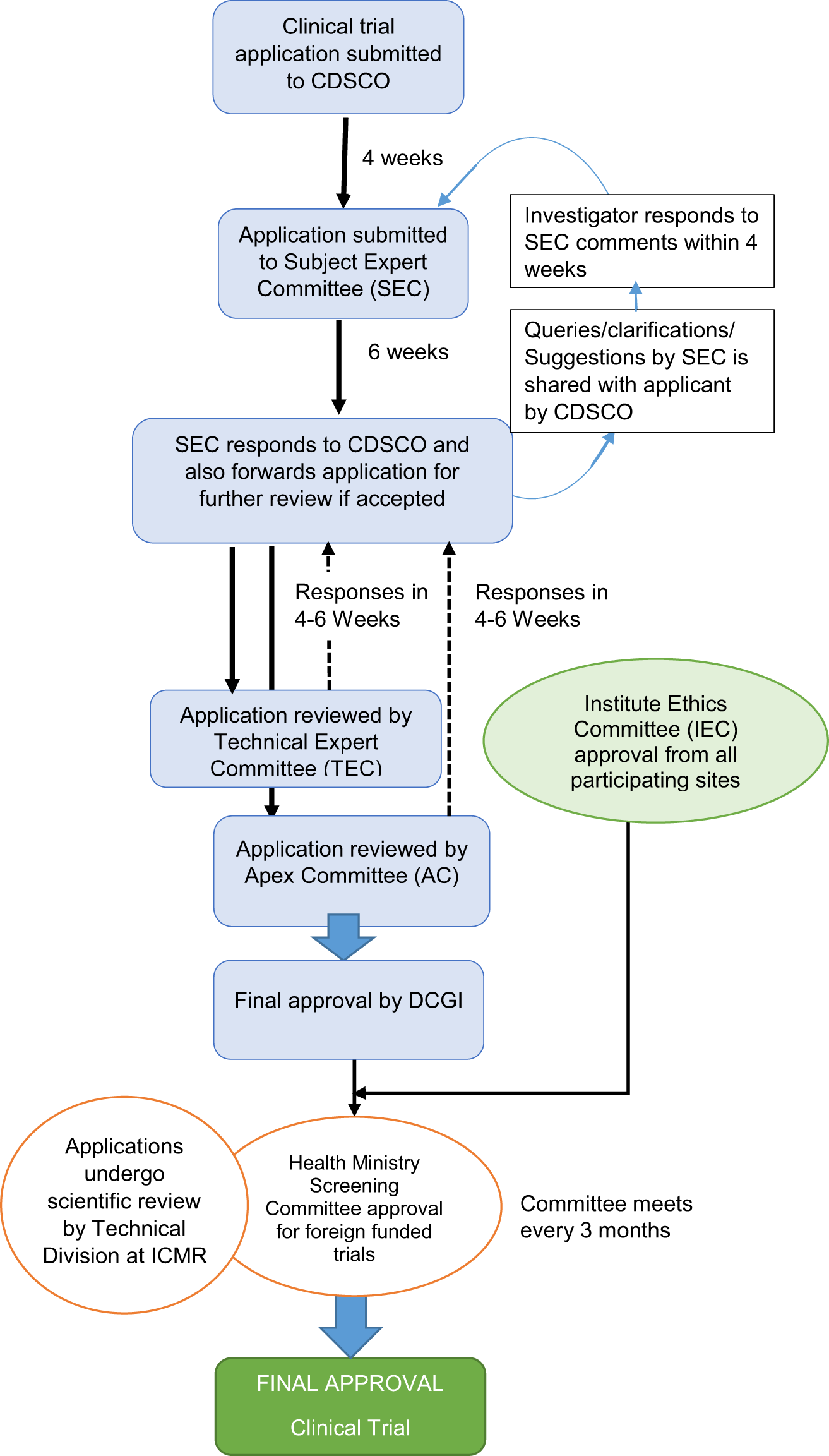
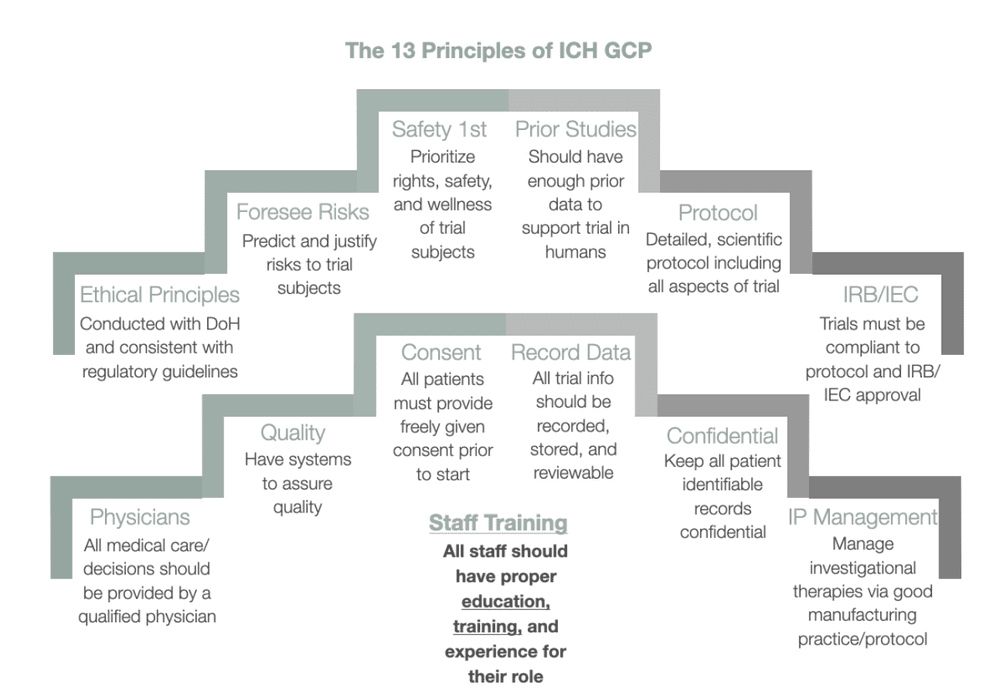
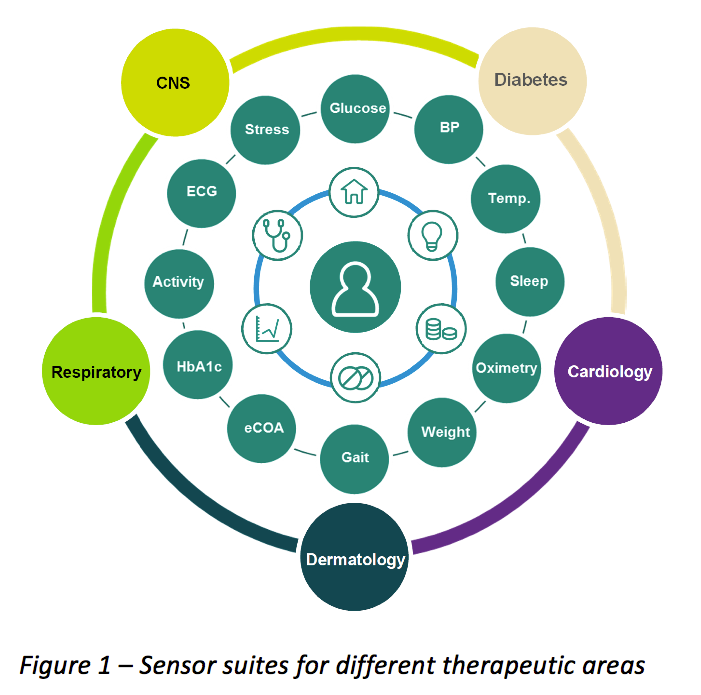
Overview of the clinical trial management structure in ICiCLe-ALL-14. A... | Download Scientific Diagram

roles and responsibilities of clinical trial personnel — Clinical Research Certification I Blog - CCRPS

Good Clinical Practice (GCP) Training and Finding | Good Clinical Practice ( GCP) Training and Finding

Fundamentals of GCP and Clinical Research: 9788192227726: Medicine & Health Science Books @ Amazon.com