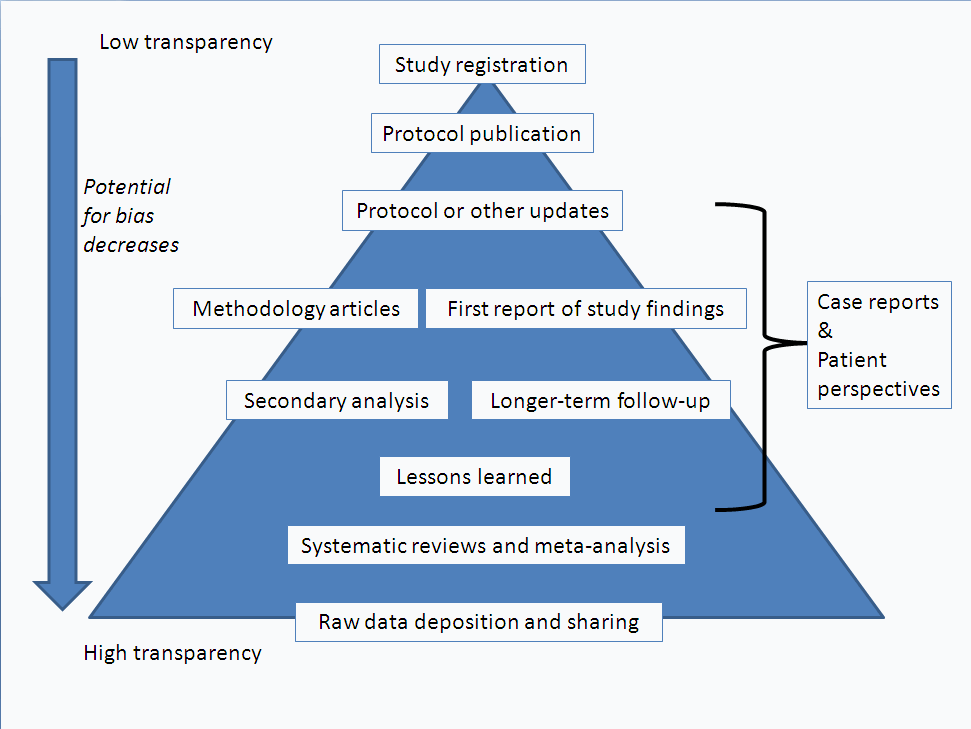
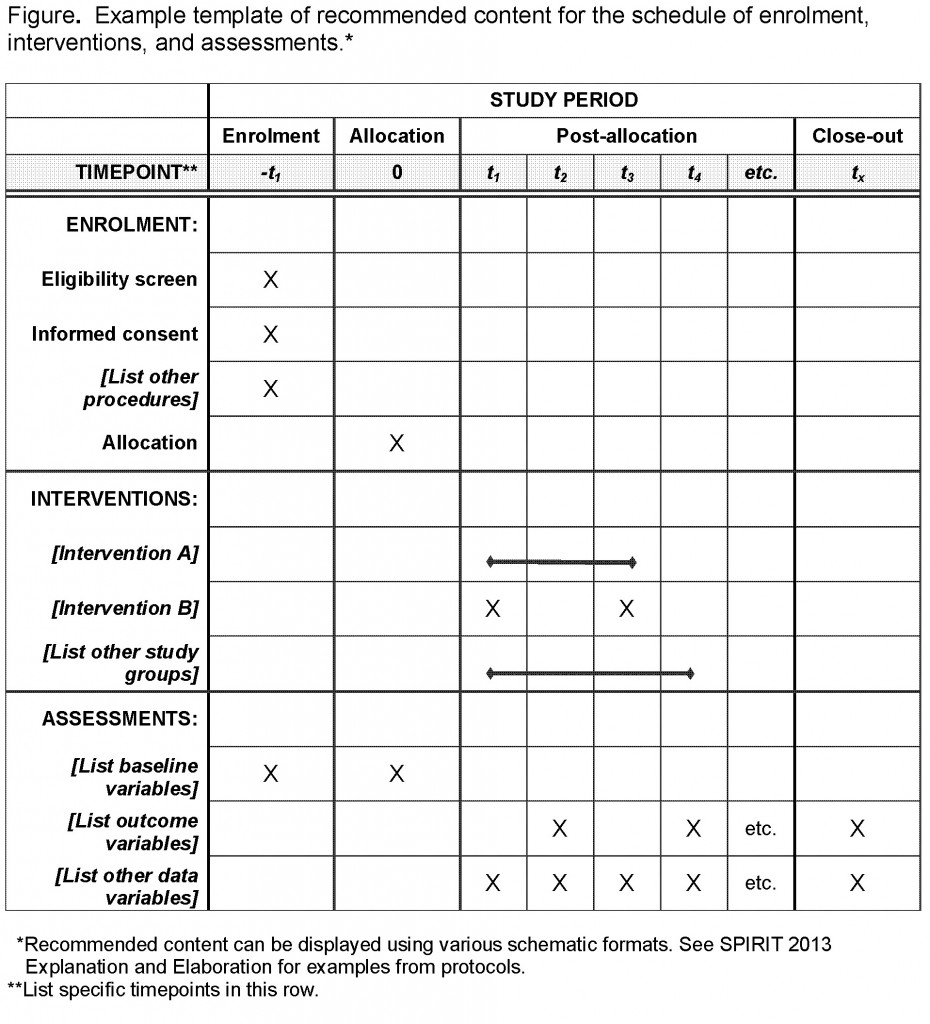
Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers | The BMJ
Evaluation of clinical trials results publications. Note: A: State of... | Download Scientific Diagram

Comparison of serious adverse events posted at ClinicalTrials.gov and published in corresponding journal articles – The Publication Plan for everyone interested in medical writing, the development of medical publications, and publication planning