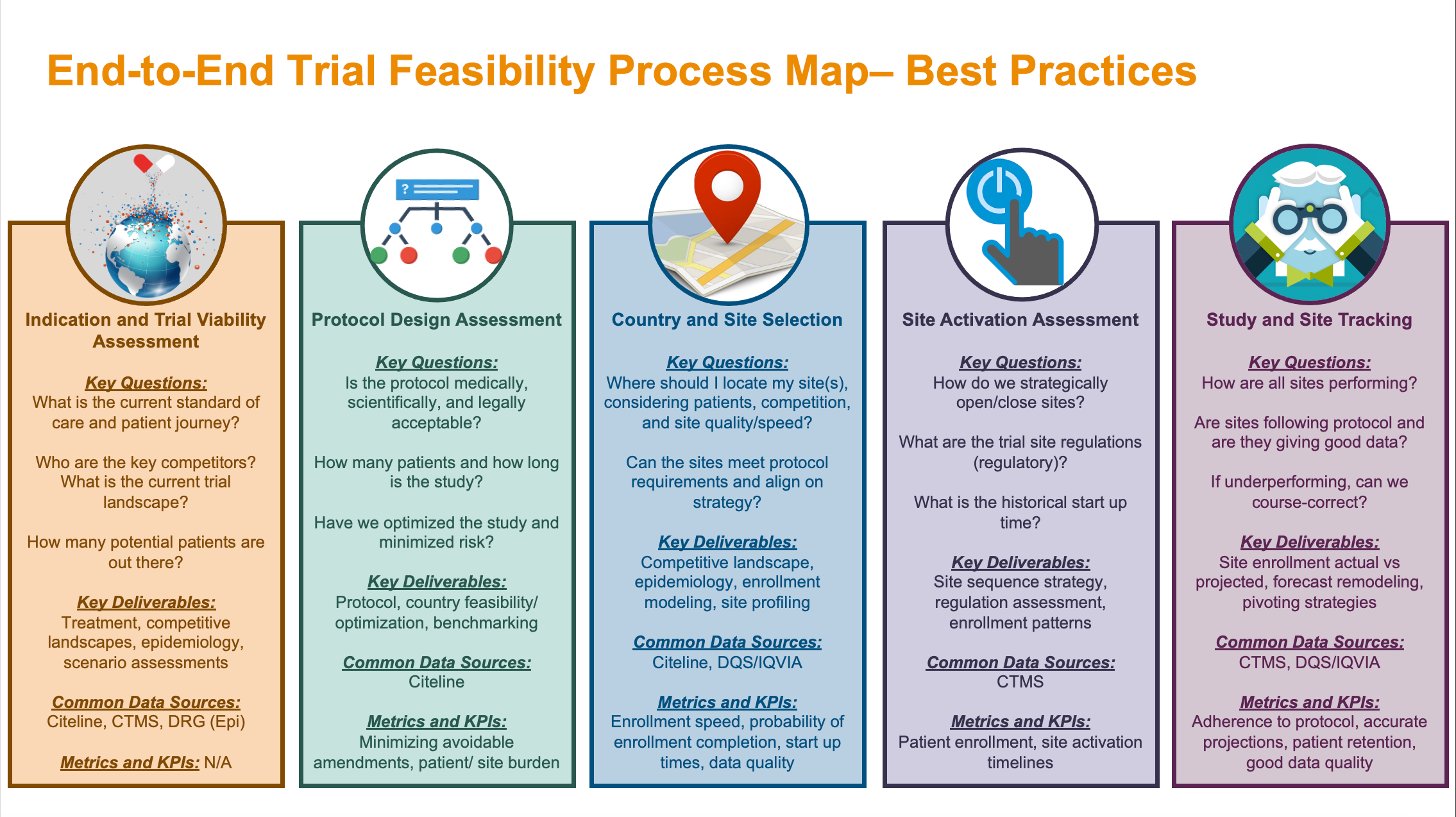
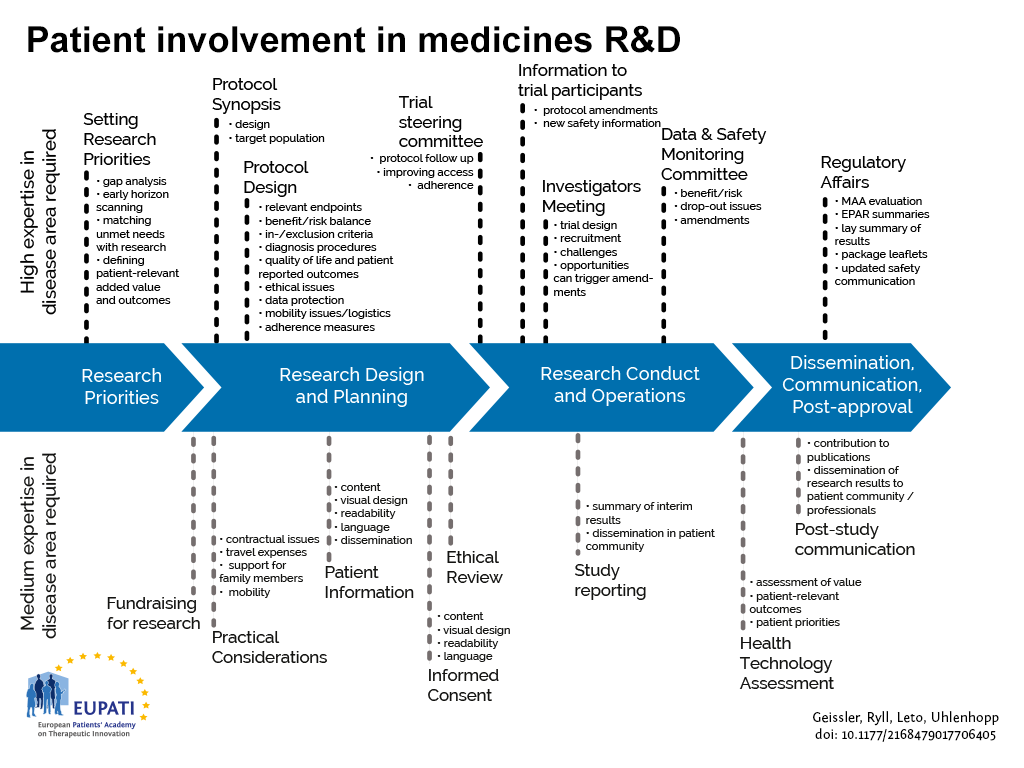
SOPs for GCP-Compliant Clinical Trials: A Customizable Manual for Sponsors of Medical Device Trials : MS Word Template | CenterWatch

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health
Guidance for Deploying Medical Image-based Software – including Algorithms – in the Clinical Setting