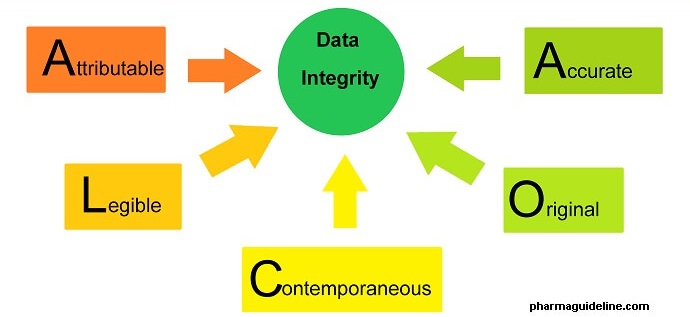
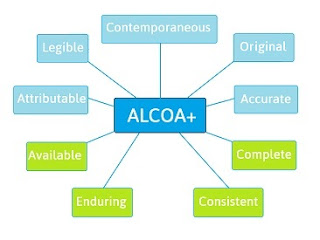
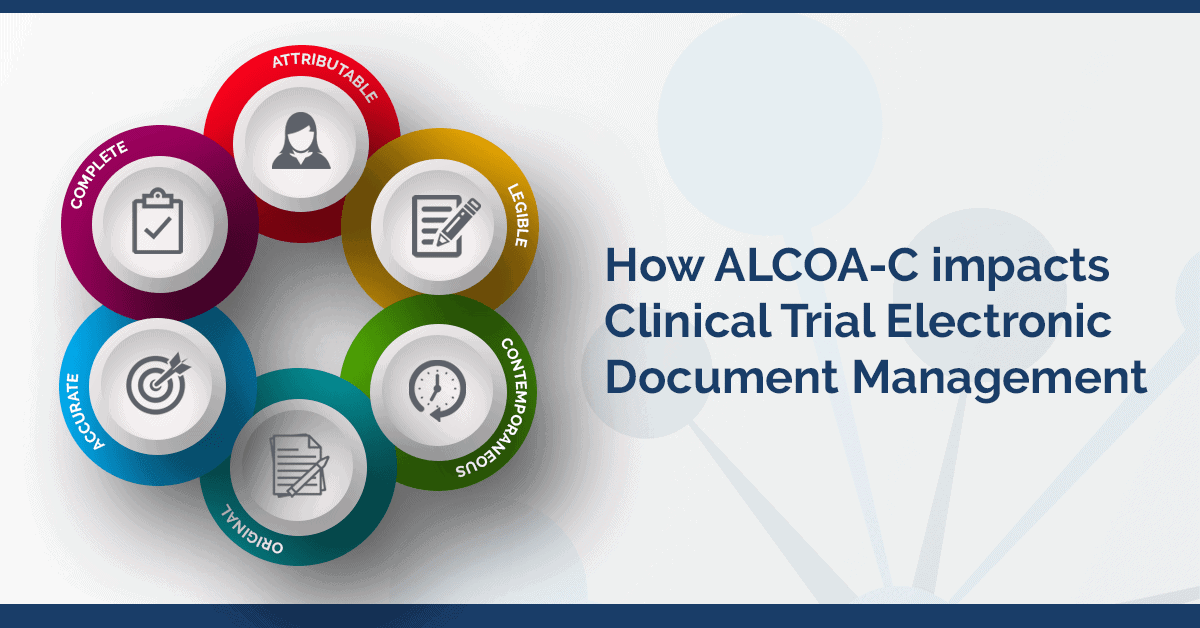
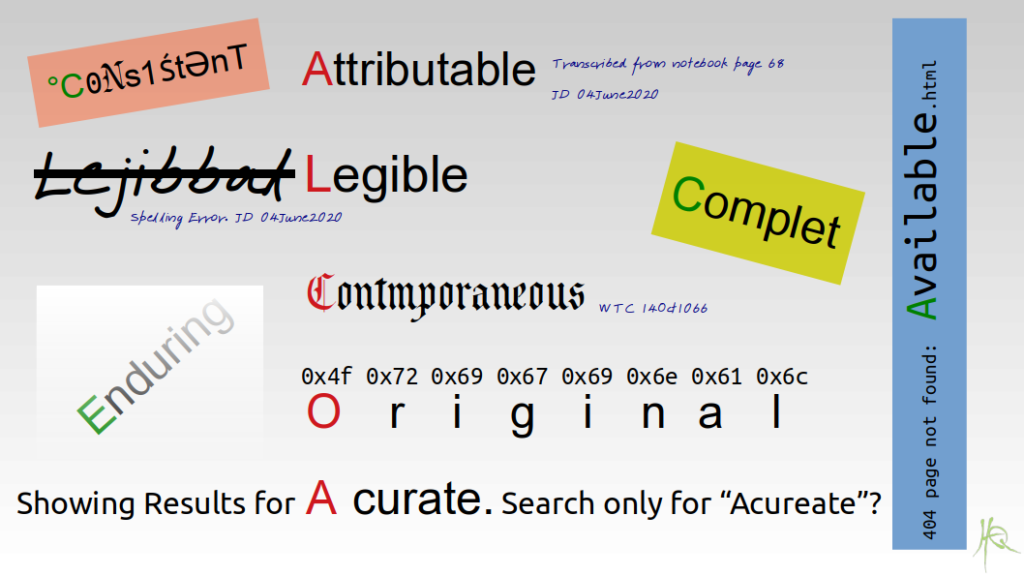
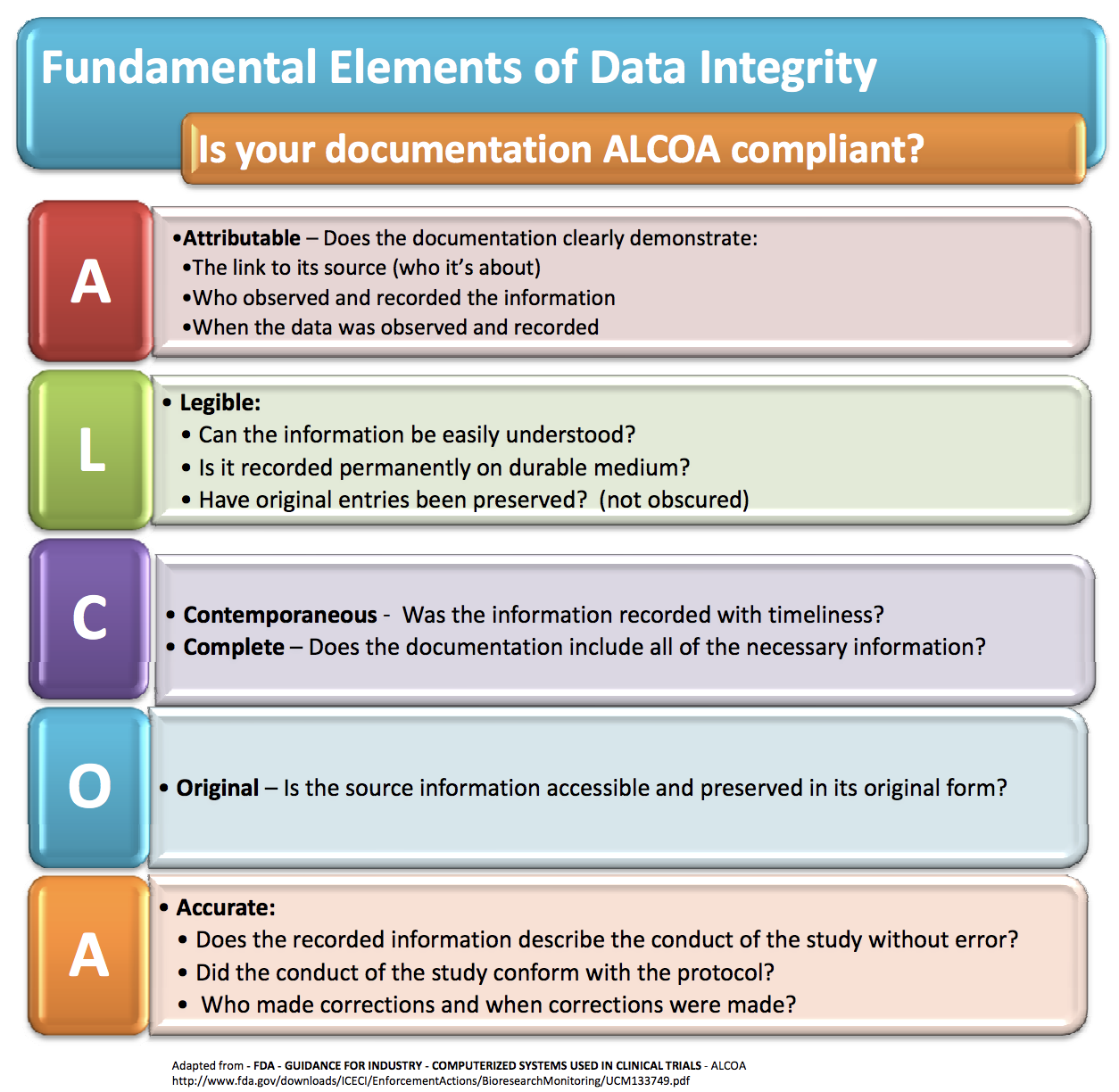
The Beginner's Guide To CRA Source Data Review and ALCOA in Clinical Research | by Dan Sfera | Medium

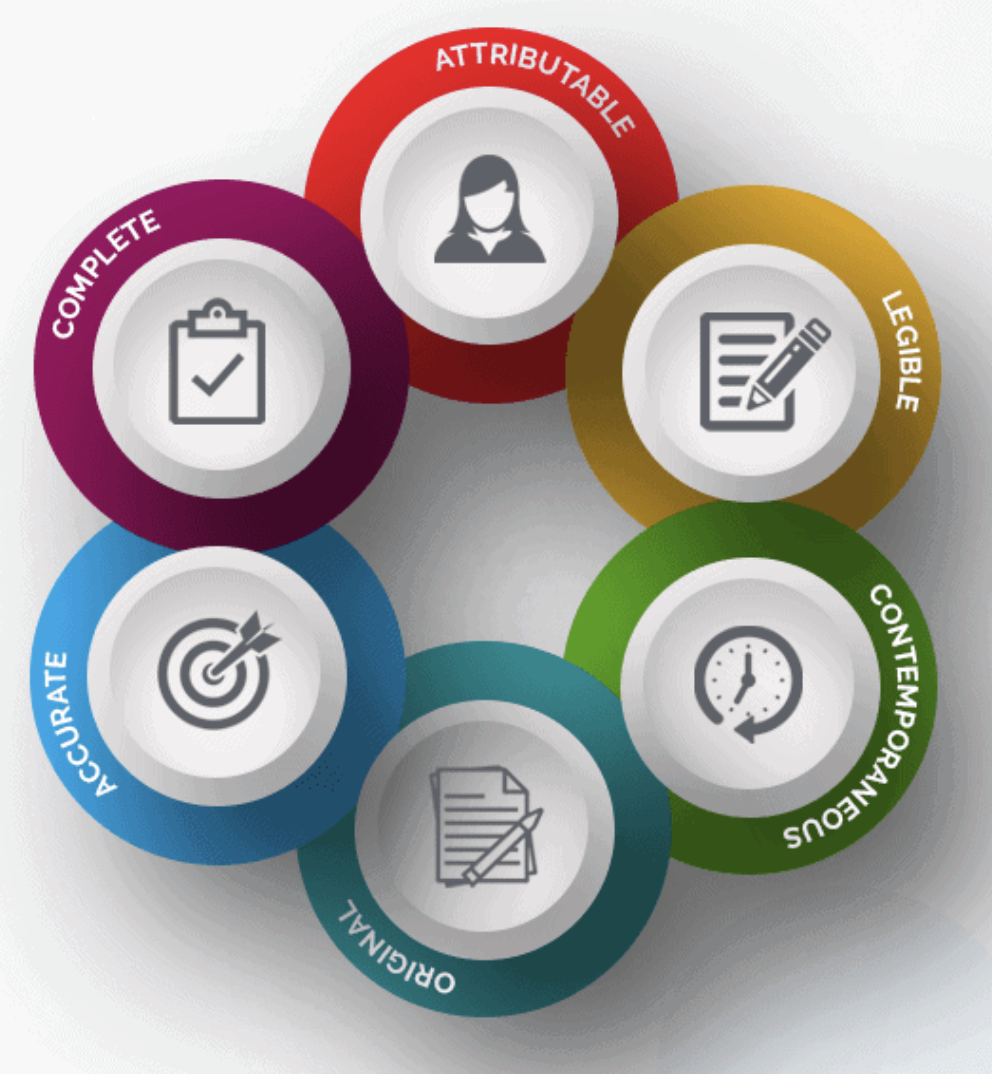
New Life Clinical Research on Twitter: "#ALCOA + C to achieve #data quality. #NewLifeClinicalResearch #nlcr #NewLifeResearch #NLCR #nlcresearch #alcoac https://t.co/RWUMbEuuvy" / Twitter